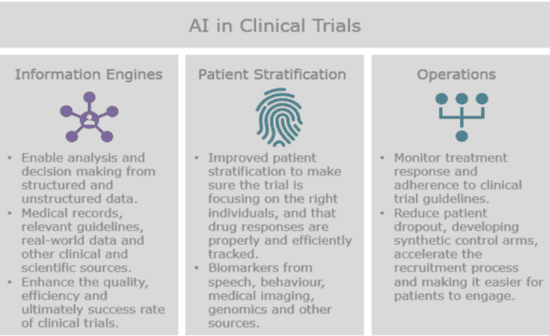
A big part of doing clinical trials is to aggregate and mine data from a number of disparate sources to enhance the efficiency, quality and success rate of clinical trials. This is done by extracting structured and unstructured data that’s relevant to the design and conduct of the trial. Natural language processing can help us to extract and analyze the information we need from a patient’s EHR records, to compare it with the eligibility criteria for all ongoing clinical trials and to recommend studies that they might be able to participate in. Extracting information from EHRs, labs, images and other medical records is one of the most highly anticipated use cases of AI in the healthcare industry.
The problem is that the solutions which are designed to do this face a range of challenges, such as disparate data sources and unstructured data. Many of the tools that standard NLP algorithms rely on, such as sentiment analysis and word sense disambiguation, struggle with clinical notes because they’re often full of acronyms, misspellings and technical language. Then there’s the fact that structured data can become unstructured due to the way that it’s transmitted. For example, if we take a spreadsheet and turn it into a PDF, it loses much of its structure, making it more difficult for researchers to collect the data that they need to determine patient eligibility.
This becomes relevant in many areas of the trials, including the design, site selection, patient recruitment and more, such as finding super-responders and evidence for new therapeutic approaches. All of these activities will benefit from us being able to analyze clinical information and figure out what and whom to focus on.
Antidote.me has made the information for clinical trials machine-readable and easy to search. It started out by gathering thousands of clinical studies from the World Health Organization and ClinicalTrials.gov, and then it hired experts to manually standardize all of that data into structured language that an algorithm could understand. Models were then trained to categorize and identify studies. Antidote has annotated more than 14,000 trials—about 50 percent of what’s listed on ClinicalTrials.gov—spanning 726 conditions. This is vital work because poor study design has hugely negative impacts on costs, efficiency and success rates.
AI tools can help us to select the optimal primary and secondary endpoints in clinical studies to define the most relevant protocols for patients, payers and regulators. The goal is to optimize study design by identifying the best possible country and site strategies, patient recruitment and startup plans and enrollment models. Better designed studies inherently lead to more predictable results, reduced development times, higher recruitment rates, fewer amendments to protocols and higher efficiency from start to finish. These improvements increase the chances of success and facilitate realistic and accurate planning.