Once you find a promising drug or device, you need to take it through trials. This is another long and complicated process. We need to make sure that these products are safe and effective before they’re prescribed to large numbers of people. So many things about this process are difficult: deciding which patient population would be appropriate, finding those patients, designing a good study protocol, recruiting the patients, ensuring that they stay with the study, collecting the right data, analyzing the data and filing it with the regulatory agency. That’s a lot, and it usually takes a long time. Life science companies have been looking for a way to improve this process, and it looks like AI could eventually be one of the catalysts to get the right products to the patients safer and faster.
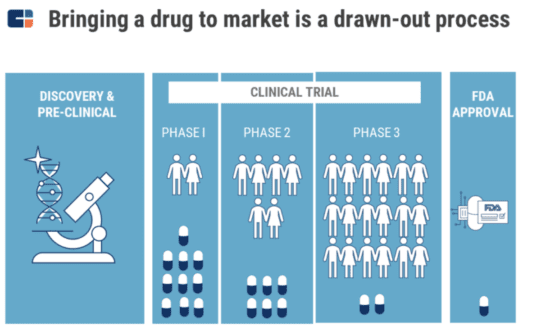
After you get through the extremely difficult and low odds process of discovery, the long and low odds process of clinical development awaits you. This is where you determine the product’s safety, the right dose and its impact on clinical outcomes. Clinical trials are carried out across multiple different phases, with their cost and complexity increasing exponentially from Phase I to Phase III. Even though significant amounts of time and money are invested in clinical trials, only 10% of the drugs that enter Phase I will go on to receive FDA approval.
There are a number of reasons why clinical trials fail, ranging from poor study design and insufficient participation from medical centers or physicians to inconsistent data, too few trial participants and patients dropping out in the middle of the trial. We also need to acknowledge that the huge costs of clinical trials have a subsequent impact on costs for patients, because biopharma companies increase the prices of their approved drugs to write off the costs of failed trials and to stay profitable.
Patients generally only participate in drug trials when existing treatments have already failed. To make matters more complicated, not all of the patients who are diagnosed are eligible to participate in trials. In fact, determining patient eligibility is a hugely difficult task on its own. Once eligibility has been ascertained, participation is often cost and time intensive. The process in general is inefficient, with drug trials lasting for an average of almost a decade and costing over $1 billion. The overall size of this market is $52 billion annually.
AI can be helpful in various parts of this process. But it’s not just AI – it’s really data and digital technologies that can transform this long and costly process. AI would be part of the solution. Life science researchers will eventually be able to use machine learning to design optimized trial protocols, identify the best locations for studies, boost patient recruitment, improve adherence to the treatment and carry out efficient and accurate data analysis.
Machine learning could also be used to speed up the process of regulatory submission, because it could help to capture and analyze the huge amounts of data that are generated during clinical trials. This data will need to be shared to enable collaboration between investigators, sponsor organizations and contract research organizations (CROs). For example, Accenture’s Intelligent Trial Planner (ITP) uses machine learning to examine forecast recruitment timelines and the overall feasibility of clinical trials. The ITP platform enables study design teams at pharma organizations to run prediction analysis in minutes, not weeks, allowing them to iterate faster and more frequently.