A technique called adaptive design, which relies on a more flexible approach to clinical trials, has become a key trend for researchers tackling COVID-19. Traditional studies are more rigid about endpoints and dosing regimens, while adaptive designs allow researchers to make modifications as the trial progresses.
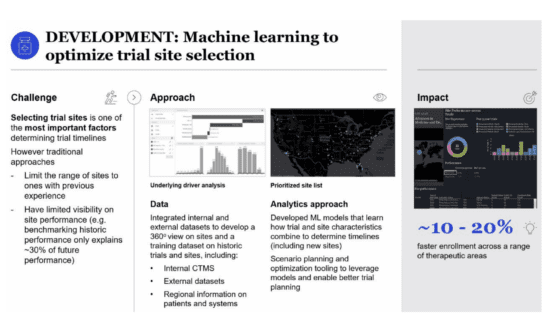
One of the biggest ongoing challenges is that of identifying trial sites with access to enough patients who meet inclusion criteria. As studies continue to target more specific populations, recruitment goals become harder and harder for them to meet. This increases costs, lengthens timelines and adds to the risk of failure. The Tufts Center for the Study of Drug Development (CSDD) says that nearly half of all sites miss their enrollment targets, while only 3% of cancer patients are currently enrolled in clinical trials. As many as 80% of all clinical trials miss their enrollment timelines, while about a third of Phase III trials are halted due to the challenge of enrollment.
Fortunately, artificial intelligence and machine learning can help us to cut down on these risks by identifying the best sites and the recruitment strategies that are most likely to work. This requires them to map patient populations and to spot the sites with the highest amount of potential for delivering a large number of patients before any of the sites are ever opened. This would allow sponsors to open fewer sites, to speed up the recruitment process and to cut down on under-enrollment.
Real-time scenario planning can be powered by machine learning and used to facilitate smarter trials by helping researchers to pick the best sites, countries and protocols to follow. By cutting down on wastage from poor resources, researchers could reduce costs by 20%. As if that wasn’t enough, these more accurate decisions based on data can be used to carry out trials more rapidly, leading to further savings and improving patient outcomes.
Technologies like deep neutral networks, natural language processing (NLP) and optical character recognition (OCR) are already being used to format structured and unstructured data to carry out safety reviews faster and more efficiently. Companies in this category include Aetion, Concerto HealthAI, Owkin, TrinetX and COTA. Each one of these uses a combination of NLP and ML to introduce intelligent automation to the process. These information engines work with other digital and manual processes within clinical trial design and operations, guiding decisions towards better patient stratification.