If you’ve determined that the workflows will be user-friendly and the data required for the AI solution to create its output will be available in a reliable and timely manner, you’re off to a good start of the business case analysis. One of the next areas to focus on will be the economic benefits of the technology under assessment. If history has taught us anything, it’s that a fancy piece of technology will garner no interest if it does not show clear benefits in one or more areas. This could be in the form of improving patient outcomes, reducing costs, enhancing efficiencies, improving supply chains, and more. All of these benefits will result in economic benefits to the buyer and/or the entire system. Better patient outcomes will translate into the ability to attract more people to your center and boost your revenues. It can also improve reimbursement and result in better revenues. Lowering costs and improving efficiencies will improve profit margins.
One issue that has served as an impediment to the commercialization of health AI solutions has been the lack of evidence for these types of benefits. How’s that even possible? Well, most of these technologies have FDA approval (for clinical solutions) based on limited evidence, establishing that the technology works. In most cases, this does not include evidence of the real-world impact of using the AI solution. Or, if they don’t require approval, they’ve been launched for operational or administrative use cases with limited data from one or few pilots. As such, the clear evidence for the ROI for the buyer is not fully established. Given how the buyers in healthcare or life science companies are bombarded with various technologies with clever talking points, clear evidence of benefit is a must.
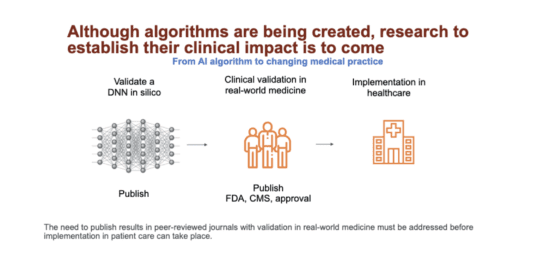
It’s absolutely critical that any new approach to the practice of medicine be fully studied to ensure that it’s safe and effective. This isn’t just an academic issue that’s insisted on by the elites who want to create a high bar for new innovation or for them to lead studies that will be done at their institutions, thus generating income for their research and departments. Many extremely promising diagnostic or treatment approaches in the past haven’t resulted in any benefits or have even harmed patients, and only well-designed trials uncovered these issues. Evidence-based medicine revolves around conscientiously and judiciously using the evidence that’s currently available to make decisions about healthcare practices. We need a similar paradigm if we’re to deploy evidence-based AI on the healthcare front line.
My discussions with many of the experts in the field have highlighted the fact that well- designed, large-scale, multi-center trials are yet to be carried out. These types of trials would establish the efficacy and safety of these algorithms in real-world settings where there are different types of patients. Also, the algorithm would be tested to see whether it could generate its output during normal clinical workflows while staying timely and being based on realistically available inputs (data.) Another challenge is that algorithms are constantly being improved and modified, so the algorithm that was tested might not be the same as the one that finally makes it into production.
A key reason for why these studies haven’t been carried out is that it costs a lot of time and money, and so they’re beyond the means of research centers and small companies. For larger companies to invest capital, they’ll need a clear path to a return on investment, and that’s not as clear for algorithms as it is for drugs and medical devices. Intellectual property protections aren’t very strong for algorithms and the barriers to entry are low.