AI can introduce key intelligent automation to different processes in the actual running of trial operations. Some examples of this include using AI in monitoring medication adherence, creating digital twins and synthetic arms that help reduce the number of patients needed, and identifying optimal patients for recruitment by analyzing clinical information.
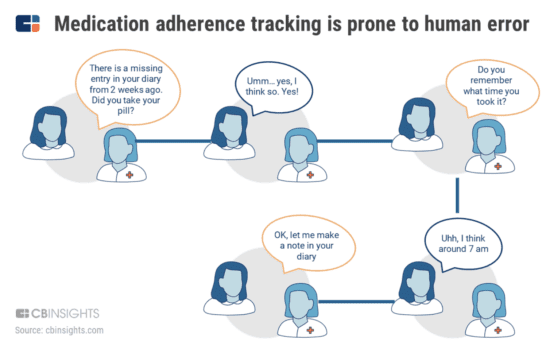
Non-adherence often leads to adverse effects on patients’ health, and it can also boost costs by forcing researchers to find new patients or compromising the accuracy of their studies. As a general rule, therapeutic efficacy is proven with adherence rates of 80% or above. The problem is that as many as half of the medications prescribed in the US aren’t taken correctly. That’s why the sponsors of clinical studies are investing in emerging technologies that could cut down on non-adherence (Figure.) Patients are being asked to note when they’re taking the drugs, what other medications they’re taking and any adverse effects that they’re experiencing, such as stomach aches, muscle aches and headaches.

This process is adversely affected by issues such as reliance on patient memory, outdated recording systems and the risk of dropout. AiCure uses an interactive medical assistant (IMA) which is designed to identify patients that are most at risk of non-adherence. The patients shoot a video of themselves taking the pill, and AiCure confirms that the right person is taking the right pill. Then there are other emerging technologies, like speech analytics and digital phenotyping, which are being used to assess adherence to medication. Mental health startup Mindstrong uses digital phenotyping technology to measure mood based on how users interact with their mobile device.
Aural Analytics uses speech detection to pick up on subtle changes to users’ brain health, and the company recently partnered with Mass General Hospital to carry out a trial for amyotrophic lateral sclerosis (ALS). As part of this, they’re using connected devices to facilitate the real-time study of medication adherence. For example, optimize.health (previously Pillsy) launched a smart medication bottle with a corresponding mobile application that provides reminders, educational content, dose tracking and patient-reported data for providers.
Unlearn.ai is being used to reduce the number of subjects that we need for clinical trials through the use of digital twins as a type of synthetic control group. This cuts down on patient resistance to the trial because it reduces the chance of them being given the placebo. This also reduces the number of patients that are required for a trial to be carried out. Synthetic data can also provide us with data that imitates real-world data, offering a potential solution to the problem of not being able to find enough patients. Computer-generated synthetic data can be used instead of manually collecting and labelling data from the real world. The goal here is to speed up the training for AI and machine learning models, driving greater cost efficiencies and bypassing the constraints that come from real world data collection. This type of data comes in different forms including demographics, labs, imaging and more. And because the synthetic data is created by a computer, it’s not based on real data or health records. Companies like Simerse create synthetic data to train AI and machine learning models.